HEARO: world’s first cochlear implant surgical robot receives CE-Mark
May 01, 2020 — (Bern, Switzerland / Innsbruck, Austria)— An exciting milestone in the future of cochlear implant surgery: HEARO, the world’s first image-guided robotic surgery platform for cochlear implants, has received CE-Mark approval. The HEARO platform is a joint initiative between leading hearing implant manufacturer MED-EL (Innsbruck, Austria) and medical technology company CAScination AG (Bern, Switzerland).
More than 20 patients have received a cochlear implant using the minimally invasive HEARO surgical procedure so far. Now, with CE-Mark approval, HEARO will be expanded to a number of cochlear implant centers across Europe for the next phase in clinical
development and ongoing research.
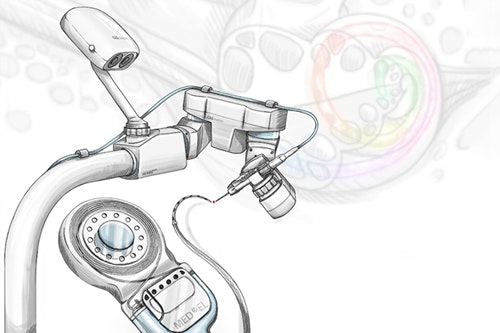
From the first HEARO procedure in Bern and the first inner ear access with HEARO in Antwerp, to becoming the first and only image-guided CI surgical robot with the CE-mark, HEARO has continued to show very promising progress in leading a whole new era
in cochlear implant surgery.
What sets HEARO apart from traditional cochlear implant surgery? With HEARO, advanced image-guided surgical planning software (OTOPLAN) helps identify
an optimal trajectory to access the round window of the cochlea.
HEARO uses this precise image-guidance and active monitoring to create a precise 1.8 mm tunnel access to the inner ear.
With image-guided direct access to the inner ear, HEARO takes minimally invasive surgery to a whole new level.
About CAScination
CAScination AG, Switzerland is an award-winning medical technology company with a mission to use transformational technology to deliver certainty in patient outcomes. CAScination’s robotic and image-guided solutions enable physicians to perform reproducible and efficient treatments and provide patients with quality outcomes from minimally invasive therapies.
To date, almost 4,000 patients with liver, lung or kidney cancer were treated with its Quality Ablation® approach, enabled by advanced computer assisted and image guidance medical technology. Quality ablations are minimal-invasive ablation treatments providing best outcomes and optimal quality of life.
CAScination’s latest product platforms, OTOPLAN and HEARO, expand the company’s reach into image-guided robotic precision surgery for cochlear implants. www.cascination.com
About MED-EL
MED-EL Medical Electronics, a leader in implantable hearing solutions, is driven by a mission to overcome hearing loss as a barrier to communication. The Austrian-based, privately owned business was co-founded by industry pioneers Ingeborg and Erwin Hochmair, whose ground-breaking research led to the development of the world’s first micro-electronic multi-channel cochlear implant (CI), which was successfully implanted in 1977 and was the basis for what is known as the modern CI today. This laid the foundation for the successful growth of the company in 1990, when they hired their first employees. To date, MED-EL has grown to more than 2,200 employees from around 75 nations and has 30 locations worldwide.
The company offers the widest range of implantable and non-implantable solutions to treat all types of hearing loss, enabling people in 124 countries enjoy the gift of hearing with the help of a MED-EL device. MED-EL’s hearing solutions include cochlear and middle ear implant systems, a combined Electric Acoustic Stimulation hearing implant system, auditory brainstem implants as well as surgical and non-surgical bone conduction devices. www.medel.com
CEO
Doz. DI Dr DDr med. h.c. Ingeborg Hochmair
Press contact
Patrick D`Haese
MED-EL Medical Electronics
Fürstenweg 77a
6020 Innsbruck
Austria