
We Help the World Hear Life
Waves crashing on the beach. The lyrics of your favorite song. A baby talking for the first time. The world is filled with amazing sounds. For more than 30 years, we’ve been helping people hear them.
As the leading hearing implant company, we’re passionate about bringing the sounds of life, laughter, loved ones, and music to people with hearing loss. It’s what we work for, and it’s what we believe in.
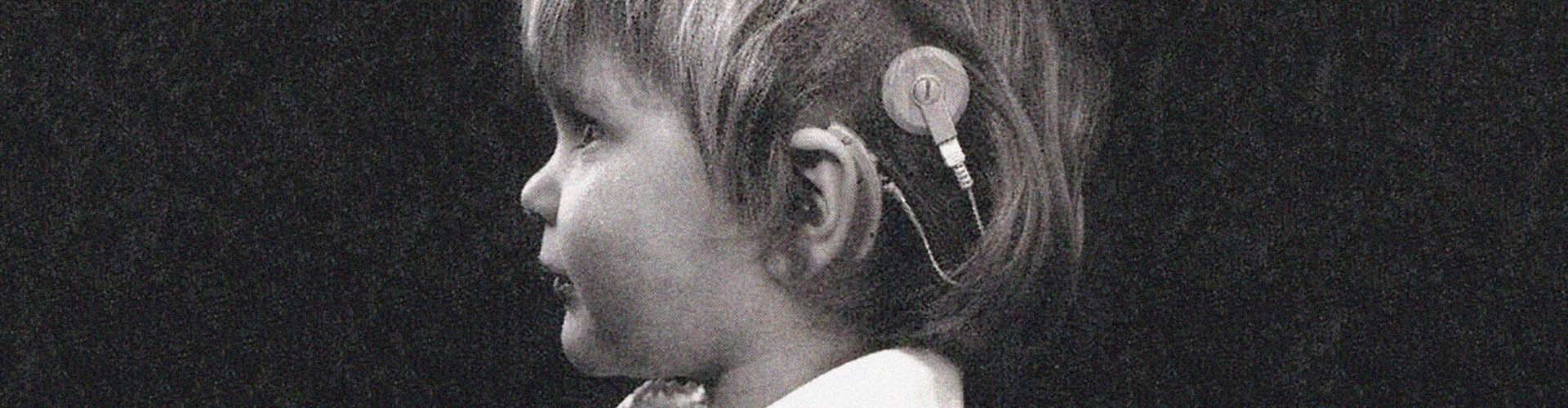
Hearing Pioneers
It’s been that way since 1977, when our CEO, Dr. Ingeborg Hochmair, pioneered the modern cochlear implant along with her husband Erwin. Since then, we have grown into a global company with over 2,700 brilliant minds from around the world, led by a management team with 450 years of experience between them. Our innovative spirit and commitment to research mean that today we offer hearing solutions for every type of hearing loss.
Worldwide Support
We’re headquartered in Innsbruck, Austria but our hearing solutions are available in 135 countries worldwide. And with more than 30 regional offices, we’re always here to offer our care and support.

Our Mission:
To help people overcome hearing loss as a barrier to communication and quality of life
A Passion to Help
Over 1.5 billion people around the world are affected by hearing loss. It limits a person’s ability to work, socialize and enjoy everyday life, and can even lead to a significant decline in mental and physical health. That’s why, at MED-EL, we’ve always been driven by one thing: A passion to help people with hearing loss.
Products You Can Trust
Our dedication to bringing people the joy of sound—whatever their type of hearing loss—means we’re the only company to offer a complete portfolio of hearing implants. And because we want to give every one of our recipients superior hearing performance and peace of mind, we make creating high-quality and reliable products a priority.
Partners in Hearing
We don’t answer to shareholders. We answer to you. That’s why we offer outstanding care and support to our hearing recipients throughout their hearing journey and treat our professional customers as partners. And it’s why we collaborate with the best clinics and universities from around the world to offer solutions that make a real difference to people’s lives.
Join Our Mission
Want to work on innovative technologies that bring the joy of sound to the world? We’re always looking for passionate and talented people to join our team.

Research
Learn how our clinical and laboratory research teams live out our mission every day to improve the lives of those with hearing loss.
Discover More
Attend an Event
Our Community Engagement Managers and HearPeers host events to help educate, support, and befriend those with hearing loss.


